

Cleanrooms provide highly controlled environments for sensitive materials and operations. In order to achieve this high level of control, they’re required to meet certain classifications and standards.
Below, we’ll give you an overview of these classifications and standards — including what they are, what different types there are, and what your application requires.
Cleanroom classifications and standards are regulations established by governing organizations in certain industries. They determine a variety of specifications and protocols related to cleanroom design, installation, and operation, including (but not limited to):
Cleanroom classifications and standards are critical in protecting the safety of personnel, products, and the surrounding environment. They’re also in place to ensure tasks are completed in a way that provides consistent, high-quality results.
Below, we’ll take a look at four of the most common types of cleanroom classifications and standards you may encounter: ISO, USP, ASTM, and GMP.
The International Organization for Standardization (ISO) established the most common standard used for cleanrooms: ISO 14644-1. This standard replaced Federal 209E standards back in 2001.
ISO 14644-1 is used to regulate the contamination level allowed in each cleanroom industry and application. It focuses on these factors specifically:
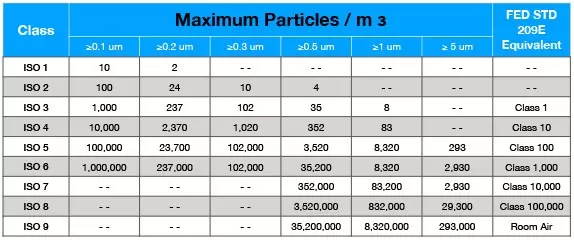
ISO 14644 is broken down into a classification system with nine different classes — each of which represents a different filtration level. ISO Class 1 is the cleanest, most filtered cleanroom and ISO Class 9 is equivalent to regular room air. Take a look at the chart below for a better understanding.
The United States Pharmacopeia (USP) has also created some standards for cleanrooms. However, their standards are specifically for pharmaceutical cleanroom applications. They were made to protect both consumer and worker safety in the development, distribution, and consumption of medicines.
The most common USP standards used in cleanroom applications are USP 797 and USP 800. USP 797 discusses requirements for pharmaceutical applications that involve compounding sterile products, such as syringes or IV medications. USP 800 discusses requirements for pharmaceutical applications that involve hazardous drugs, such as antineoplastic drugs used to treat cancer.
Since they’re more specialized, USP standards generally set a few more requirements than ISO standards. They can include anything from air filtration to workstation placement and operator training and technique.
Next is the American Society for Testing and Materials (ASTM). ASTM has created a few standards related to cleanroom design and operations, mostly for the manufacturing and industrial sectors.
ASTM standards are mainly a system of quality control. They regulate the processes done within cleanrooms to ensure consistent, high-quality results. They are especially common for automotive, aerospace, and plastics manufacturing cleanrooms.
Some examples of ASTM standards used in cleanroom settings include ASTM E2312, ASTM E2090, and ASTM E2614.
And finally, GMP. GMP stands for Good Manufacturing Practices, which is a quality management system enforced by the United States Food & Drug Administration. GMP standards are most commonly used by medical and pharmaceutical manufacturers to minimize the risks of microbiological, particulate, and pyrogen contamination during the preparation and sterilization of medicines or medical devices within a cleanroom. They address a broad range of cleanroom operation and process issues, spanning anywhere from personnel and equipment qualification to record-keeping and documentation.
Similar to how ISO 14644-1 requirements are broken into Classes 1-9, GMP standards are broken into Grades A-D.
So there you have it — a brief overview of some of the cleanroom classifications and standards you may come across. But if you’re planning to build a cleanroom of your own, you might be wondering which one(s) you need to follow.
This heavily depends on your industry and application; how sensitive the materials are, how hazardous substances may be to personnel and the surrounding environment, whether or not materials are manufactured for human consumption, etc. Based on that information, you could need to focus on a different set of standards or even different standards within that set.
For example, say you’re a pharmaceutical company manufacturing drugs to treat cancer. Your cleanroom will likely have to comply with requirements from a stringent ISO Class, USP 800, and GMP Grade A or B. But if you’re a water treatment lab testing water samples for pollution, your cleanroom may only need to comply with a less-stringent ISO Class.
Cleanroom classifications and standards can get complicated, but they’re incredibly important in ensuring your operations are consistently controlled, repeatable, and of high quality. At Starrco, we can provide a modular cleanroom solution that meets your facility’s needs and is compliant with industry standards, answering any questions you have along the way. To get started, contact our team online.